
Thursday, March 31, 2011
Happy birthday, Bunsen
I'm sure I'm not the only one who logged onto the computer this morning and wondered why Google had a bunch of lab equipment as their logo. It turns out today is the 200th anniversary of the birth of Robert Bunsen, designer of the gas flame burner we've all used in lab (see a LEGO version by Neodymium-boy below). Bunsen created this gas flame (or improved on previous designs, to be more exact) as part of his study of emission spectra of elements. When you heat up a substance, the energy can bump electrons from lower energy levels up to higher energy levels, putting them at a higher potential energy. This is analogous to lifting a weight - by putting in energy, you are putting the weight at a higher potential energy relative to the earth's gravity. When you let go of the weight, it will fall back down. The same is true of electrons; they will relax from a higher energy state to a lower energy state. That energy has go go somewhere (the conservation of energy tells us that energy is neither created nor destroyed, it simply changes forms), and in many cases it is given off in the form of light. Studying the energy of the light given off tells us about the relative energies of the electrons involved.


Thursday, March 24, 2011
Dissected frog
I have no idea how I made it through all those years of science education and never dissected anything (oh, in one biochem lab we had to isolate proteins from rat livers, but my lab partner was a biologist and she did the actual dissection). Regardless, cutting into a frog, or a fetal pig, is a common activity in both high school and college science to teach students the details of anatomy. Some have argued that the educational value of this is overrated, and have proposed virtual dissections or other alternatives. Dave Kaleta came up with another solution, a LEGO dissected frog. If you look close, you can clearly see the heart, the lungs, the liver and the spine. There are a couple of elements I don't recognize, but given the authenticity of the rest, I'm sure Dave was working to closely emulate his source material.

BTW, in looking around the web, I've seen some other interesting variations, such as a knitted frog. That one also led to another interesting blog, the Art of Science, that I'm going to have to read more closely.

BTW, in looking around the web, I've seen some other interesting variations, such as a knitted frog. That one also led to another interesting blog, the Art of Science, that I'm going to have to read more closely.
Saturday, March 19, 2011
Particle Accelerator
Shane Larson is a physicist at Utah State University, and also a LEGO hobbyist. So it's very appropriate that he built a scene of the target from a particle accelerator. He's even included a photo and diagram of the real thing so you can compare. A particle accelerator takes charged particles (like protons) and propel them at great velocities at a target. When they crash into the target, they break apart into even smaller building blocks, that can then be studied by the physicists to learn about the fundamental structure of matter.
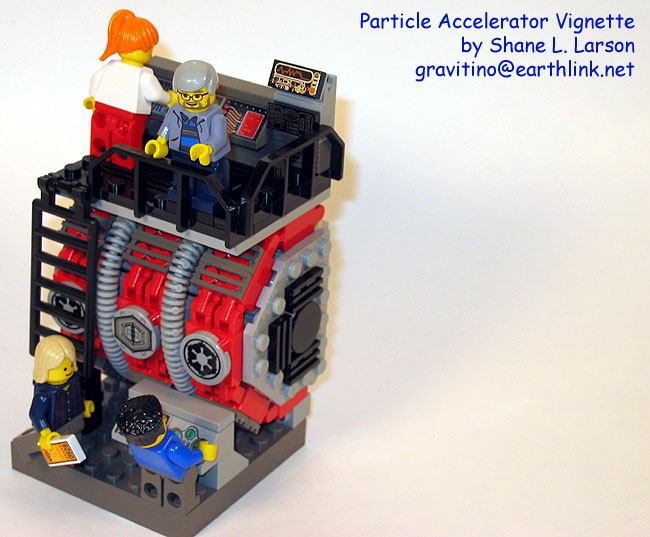
BTW, this is a vignette, or a scene built on a small base. For more vignette LEGO creations, see VignetteBricks.

BTW, this is a vignette, or a scene built on a small base. For more vignette LEGO creations, see VignetteBricks.
Thursday, March 17, 2011
Happy Saint Patrick's Day
Think green for Saint Patrick's Day, green chemistry, that is. In recent years there has been a real movement for more environmentally friendly, or 'green chemistry'. This means the development of processes that are more efficient, use more bio-friendly materials (like water as a solvent instead of something like chloroform), and produce less waste. Janet Scott, who focuses on green chemistry at Monash University in Australia has developed a series of reactions she calls moelcular LEGO, because she synthesizes molecules with activated 'male' and 'female' groups that click together like the studs and tubes of LEGO bricks. She can then connect these pieces in various ways to make much larger molecules.

I should note that she is not the first person to use LEGO as an analogy for chemical transformations. A quick search of pubs.acs.org, which is the site for those chemical journals published by the American Chemical Society, turns up over 200 hits on the word 'LEGO'. Some of these are from the Journal of Chemical Education, where actual LEGO bricks were being used for demonstrations to teach chemistry to kids, but others are research articles. There are surely similar instances in other scientific disciplines. I'm sure I'll feature many such articles here on SciBricks in the weeks and months to come.

I should note that she is not the first person to use LEGO as an analogy for chemical transformations. A quick search of pubs.acs.org, which is the site for those chemical journals published by the American Chemical Society, turns up over 200 hits on the word 'LEGO'. Some of these are from the Journal of Chemical Education, where actual LEGO bricks were being used for demonstrations to teach chemistry to kids, but others are research articles. There are surely similar instances in other scientific disciplines. I'm sure I'll feature many such articles here on SciBricks in the weeks and months to come.
Monday, March 14, 2011
Discovery
A few days ago, the Space Shuttle Discovery finished its final mission. Originally conceived in the late 1960's the Space Shuttle program was designed as a replacement for the Apollo program, that brought the first men to the moon. Unlike Apollo, the Shuttle is largely re-usable, cutting down on the expense and allowing for over 130 missions by the five Shuttle craft (two destroyed, two still in operation, and the Discovery retired this past week). These missions have included scientific experiments, deploying, servicing, and occasionally retrieving satellites, and the construction of the International Space Station (more on that in future blog posts). LEGO has released many official sets focused on space exploration over the years, including a few different variations on the Space Shuttle, including one that is specifically the Discovery, set 7470, seen here deploying the Hubble Telescope (more on the Hubble in a future blog post).


Thursday, March 10, 2011
Molecular models
I guess I should start things off with one of my own and give a little insight into myself. Chemists in general, and organic chemists in particular, often build models of the molecules that interest them. Dean Tantillo, a friend of mine who is a chemist at UC Davis, assembled a gallery of photos of chemists and their models. A model could be defined as a representation of a real object that tries to capture some of the properties of that object while abstracting other properties. For instance, a model airplane built out of LEGO might capture the basic shape and color scheme of a real airplane, but it is much smaller and does not fly. A molecular model allows us to examine the relative arrangements of atoms in a molecule, but is obviously at a significantly different scale. Nobel laureate Roald Hoffmann has a great essay on how the method we use to depict molecules affects how we think about them (compare to Orwell's thoughts about how the language we use affects how we think). His essay is more about 2-dimensional depictions, but it also applies to 3-dimensional models.
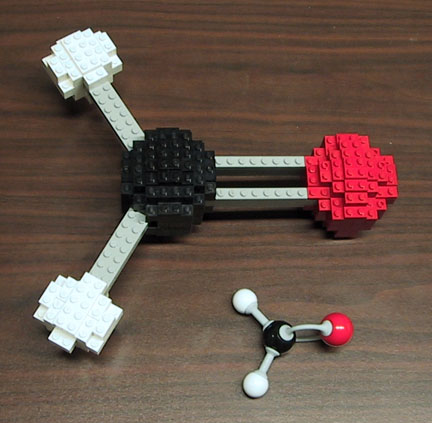
Here is a LEGO model of formaldehyde that I built, sitting next to a model using a commercially available molecular modeling set. In the color scheme that organic chemists tend to use, the black sphere represents a carbon atom, the red sphere represents an oxygen, and the white spheres represent hydrogens. The gray lines represent bonds, or shared pairs of electrons. Note that two of the bonds are single bonds and one is a double bond. Also note that the angles between the bonds are roughly 120 degrees, which is appropriate to the actual model.
On a personal note, I really do think that my building with LEGO as a kid helped contribute to my interest in science as an adult. When I took chemistry and started building models, it immediately took me back to the happy days of my youth playing with a pile of little plastic blocks. Thinking in three dimensions is very important to an organic chemist, and again I think that LEGO modeling helped develop that skill.

Here is a LEGO model of formaldehyde that I built, sitting next to a model using a commercially available molecular modeling set. In the color scheme that organic chemists tend to use, the black sphere represents a carbon atom, the red sphere represents an oxygen, and the white spheres represent hydrogens. The gray lines represent bonds, or shared pairs of electrons. Note that two of the bonds are single bonds and one is a double bond. Also note that the angles between the bonds are roughly 120 degrees, which is appropriate to the actual model.
On a personal note, I really do think that my building with LEGO as a kid helped contribute to my interest in science as an adult. When I took chemistry and started building models, it immediately took me back to the happy days of my youth playing with a pile of little plastic blocks. Thinking in three dimensions is very important to an organic chemist, and again I think that LEGO modeling helped develop that skill.
Welcome to SciBricks
Hi all,
Welcome to SciBricks. In my professional life, I'm a chemist; specifically I teach organic chemistry. For fun I like to collect LEGO sets. This blog will try to bring together those two aspects of my world. Instead of simply showing cool LEGO models (there are lots of blogs that do that), I'm going to try to teach something about science along the way. My goal is to show that science can be fun. Hopefully I will encourage some kids to pursue further studies in the sciences, and even inspire adults to think about some subjects that they haven't pursued since they were students.
Welcome to SciBricks. In my professional life, I'm a chemist; specifically I teach organic chemistry. For fun I like to collect LEGO sets. This blog will try to bring together those two aspects of my world. Instead of simply showing cool LEGO models (there are lots of blogs that do that), I'm going to try to teach something about science along the way. My goal is to show that science can be fun. Hopefully I will encourage some kids to pursue further studies in the sciences, and even inspire adults to think about some subjects that they haven't pursued since they were students.
Subscribe to:
Posts (Atom)