Wednesday, August 3, 2011
Periodic Table of the Elements
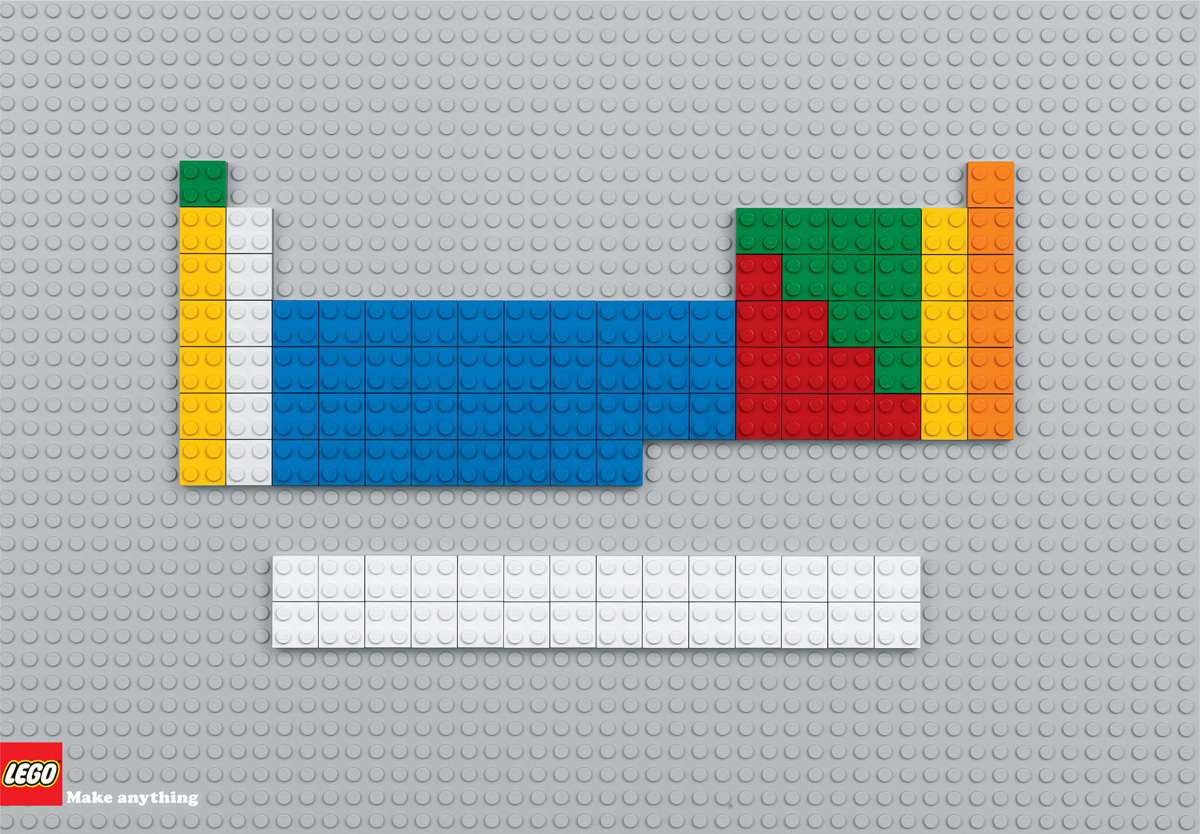
Surely everyone recognizes the periodic table, posted on the wall of every chemistry classroom, but what does it mean and does that characteristic shape bear any meaning? Let's start with the basics - The world around you is made up of a little over 100 fundamental types of atoms, called elements. These elements are characterized by the number of protons in the nucleus - Hydrogen, the smallest element, has only one proton, Helium has two, Lithium has three, and so on. Mendeleev noticed that when you arrange these by increasing number of protons, there are repeating properties - every eighth element reacts in a similar way. It turns out, reaction patterns are related to the arrangement of electrons around the nucleus. These electrons are found in regions called orbitals. S orbitals hold two electrons, p orbitals hold six, d orbitals hold ten and f orbitals hold 14. Now take a close look at the table below. On the left=hand side is a block made of two columns, then a block colored blue made up of ten columns, then another block made of six columns, and down below is a white section made of fourteen columns. That's not accidental. Neutral atoms have an equal amount of protons and electrons, so Mendeleev's list could also be characterized by an increasing number of electrons. Reading from left to right and moving down the table line to line, the first two electrons are in s orbitals, the next two are also in s orbitals, then the next six electrons go into p orbitals, and so forth.


Labels:
chemistry
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment