
Wednesday, August 31, 2011
Bye-bye, figs!
Pcdos61 was there to watch the launch of the Juno probe (the one that is carrying three minifigs to Jupiter).


Wednesday, August 17, 2011
Fermat, well, no, Pythagoras
If Google is your home page (it's mine), you noticed this morning that today is the 410th anniversary of Fermat's birth. We all remember from high school geometry that the sum of the squares of the sides of a right trangle equals the sum of the hypotenuse, or a2 + b2 = c2, the Pythagorean theorem. Fermat famously came up with his 'last theorem', that this did not work for other powers, e.g. there are no positive integers that lead to a3 + b3 = c3. Unfortunately he didn't have space in the margin where he noted to give the proof. Or maybe that was fortunate, as it inspired the last few centuries of mathematicians to tackle this problem, until it was finally proven in 1995. I couldn't find any LEGO creations relevant to Fermat, but the Pythagorean theorem is useful in building with LEGO. You may think that LEGO is limited to square structures, but using pythagoras you can connect things at other angles, using 3/4/5 triangles, or 5/12/13, etc. For example:

Or you can see the lines here:
The late Erik Brok did some work on this using hinges:

and technic beams:

There's more discussion of how to use Pythagoras in LEGO building on various LEGO sites, like here, here and . Break away from the rigid rules of right angles!

Or you can see the lines here:

The late Erik Brok did some work on this using hinges:

and technic beams:

There's more discussion of how to use Pythagoras in LEGO building on various LEGO sites, like here, here and . Break away from the rigid rules of right angles!
Monday, August 15, 2011
What hath God wrought?
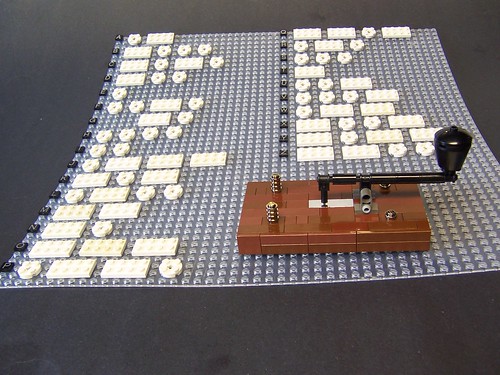
During the early 1800's there were a series of inventions designed to transmit messages over wires, leading up to the point where Morse developed his telegraph (here in LEGO by Monsterbrick) in 1837. This was the first step of the telecommunications revolution that brought the world together, leading up to the internet today.

Thursday, August 4, 2011
More on Juno
The Brothers Brick had some additional links on the figs going to Jupiter - the press releases are available from NASA and LEGO.
LEGO writes that "The LEGO crew’s mission is part of the LEGO Bricks in Space project, the joint outreach and educational programme developed as part of the partnership between NASA and the LEGO Group to inspire children to explore science, technology, engineering, and mathematics." and that information and activities will be found at LEGOspace.com.

BTW, looking closely at the figs, I notice that Galileo isn't just holding a simple ball, but instead it's engraved to look like Jupiter (you can see the swirling clouds and the great red spot).
LEGO writes that "The LEGO crew’s mission is part of the LEGO Bricks in Space project, the joint outreach and educational programme developed as part of the partnership between NASA and the LEGO Group to inspire children to explore science, technology, engineering, and mathematics." and that information and activities will be found at LEGOspace.com.

BTW, looking closely at the figs, I notice that Galileo isn't just holding a simple ball, but instead it's engraved to look like Jupiter (you can see the swirling clouds and the great red spot).
Wednesday, August 3, 2011
Minifigs headed to Jupiter
Three minifigs are headed for Jupiter! These three stowaways, representing Jupiter, Juno and Galileo, are affixed to the Juno space probe, which is launching in two days. The probe will arrive near Jupiter in July 2016. LEGO carved these out of solid aluminum to include in the probe. They only revealed this little detail at the last minute - I hope they actually make something out of this and produce these as plastic figs. I'd love to own an official Galileo fig.


Periodic Table of the Elements
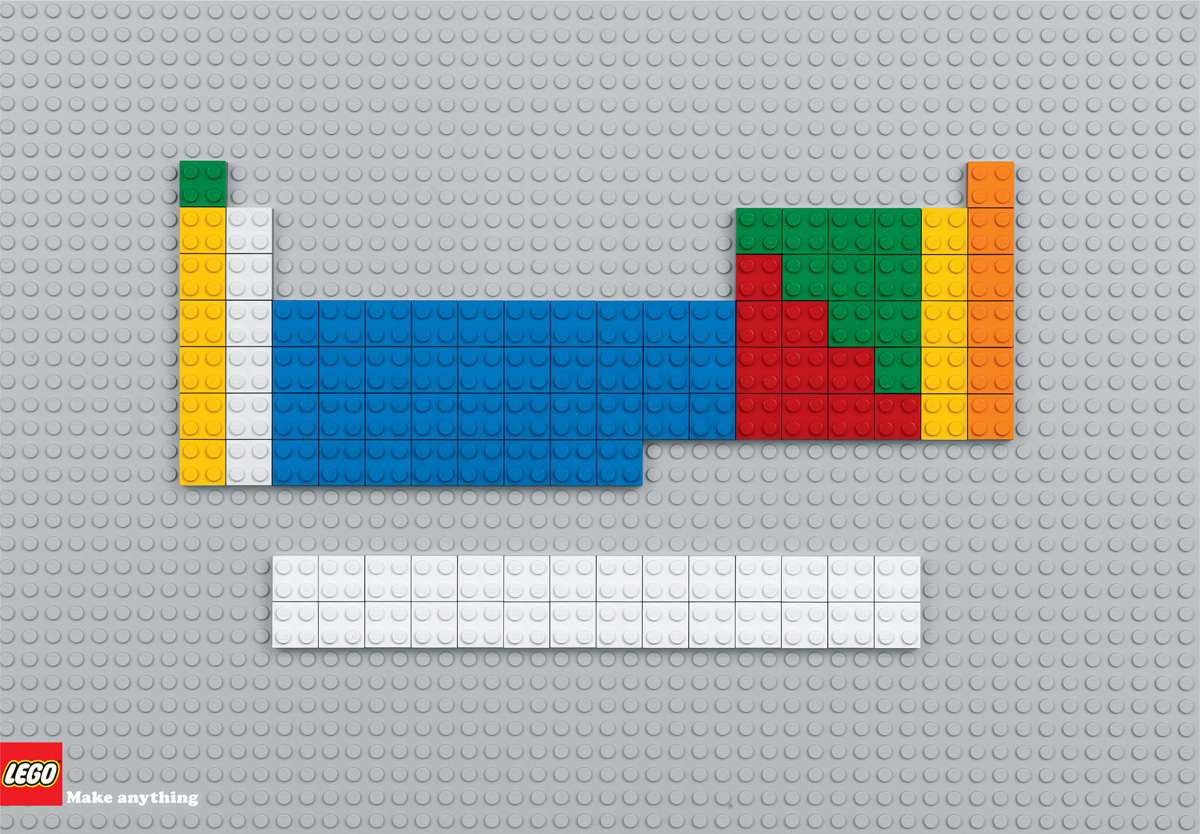
Surely everyone recognizes the periodic table, posted on the wall of every chemistry classroom, but what does it mean and does that characteristic shape bear any meaning? Let's start with the basics - The world around you is made up of a little over 100 fundamental types of atoms, called elements. These elements are characterized by the number of protons in the nucleus - Hydrogen, the smallest element, has only one proton, Helium has two, Lithium has three, and so on. Mendeleev noticed that when you arrange these by increasing number of protons, there are repeating properties - every eighth element reacts in a similar way. It turns out, reaction patterns are related to the arrangement of electrons around the nucleus. These electrons are found in regions called orbitals. S orbitals hold two electrons, p orbitals hold six, d orbitals hold ten and f orbitals hold 14. Now take a close look at the table below. On the left=hand side is a block made of two columns, then a block colored blue made up of ten columns, then another block made of six columns, and down below is a white section made of fourteen columns. That's not accidental. Neutral atoms have an equal amount of protons and electrons, so Mendeleev's list could also be characterized by an increasing number of electrons. Reading from left to right and moving down the table line to line, the first two electrons are in s orbitals, the next two are also in s orbitals, then the next six electrons go into p orbitals, and so forth.

Subscribe to:
Comments (Atom)