Monday, May 27, 2013
Formaldehyde
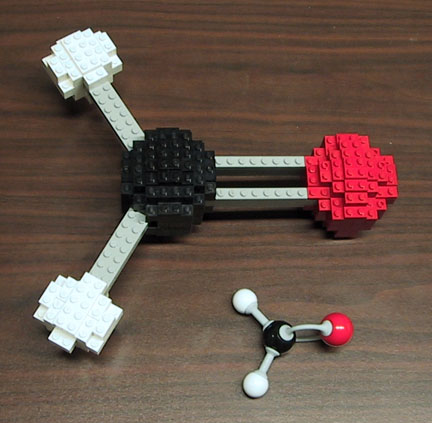
You might have noticed that I'm making some slight changes to the look of this blog, including the graphics added to the banner at the top of this page. I tried to choose some models from a few (but by no means all) of the major disciplines covered on this blog - chemistry, math, biology and astronomy. Let's take a few blog posts to look at the models I used. Starting at the left end with one of my own, here is my model of formaldehyde (shown next to a standard organic chemistry model kit version). Formaldehyde is made up of one atom of carbon (shown in black), with a double bond to an atom of oxygen (red) and two bonds to hydrogen atoms (white). One thing that is demonstrated by this model is the shape of formaldehyde. Molecular shape is driven by VSEPR - Valence Shell Electron Pair Repulsion theory. The bonds (gray lines) are made up of negatively chaged electrons, and, since like charges repel, those bonds push each other away. VSEPR says that the best (lowest energy) arrangement of bonds will maximize the distance between the bonds. Here we have three sets of bonds around carbon, and the furthest they can get from each other is 120 degree angles (360 / 3).


Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment